Difference between revisions of "Matrix Acidizing"
| Line 1: | Line 1: | ||
| + | |||
| + | Calcite - DOES react to HCl | ||
| + | Dolomite | ||
| + | Siderite | ||
| + | Ankerite | ||
| + | Feldspar - DOES NOT react to HCl | ||
4HF+SiO2→↑SiF4+2H2O | 4HF+SiO2→↑SiF4+2H2O | ||
Revision as of 10:27, 20 May 2019
Calcite - DOES react to HCl Dolomite Siderite Ankerite Feldspar - DOES NOT react to HCl
4HF+SiO2→↑SiF4+2H2O
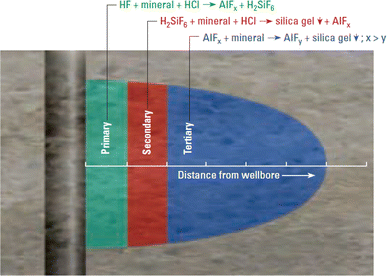
Acidizing mechanism HF acid starts dissolution of minerals as soon as it enters a sandstone formation. The speed of reaction and dissolution of minerals depends on their reaction rate with acid and the exposed surface areas. The sandstone minerals are divided into two different categories: slow and fast reacting. “Quartz tends to act at a slower rate whereas feldspars and clays tend to react at a faster rate” (Ponce da Motta et al. 1992). Figure 2 shows the types of reactions occurred when sandstone formation is exposed to mud acid.